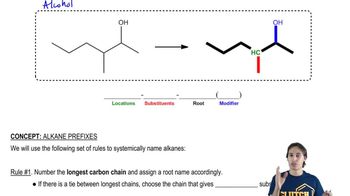
3,4-Dimethylpent-1-ene has the formula CH2=CH—CH(CH3)—CH(CH3)2. When pure (R)-3,4-dimethylpent-1-ene is treated with hydrogen over a platinum catalyst, the product is (S)-2,3-dimethylpentane.
c. The reactant is named (R), but the product is named (S). Does this name change imply a change in the spatial arrangement of the groups around the chiral center? So why does the name switch from (R) to (S)?