Textbook Question
What is the role of a solvent in a chemical reaction? What happens to the solvent at the end of a reaction?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: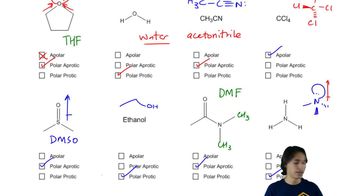
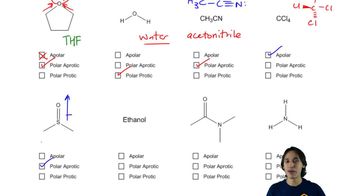
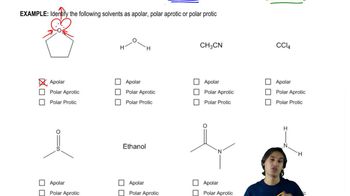

 2:26m
2:26mMaster General format of reactions and how to interpret solvents. with a bite sized video explanation from Johnny
Start learning