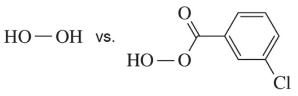
When producing a chiral molecule, epoxide formation still results in a mixture of enantiomers, despite its stereospecificity.
(b) How is it that a reaction can be stereospecific while still producing two enantiomers?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 2:19m
2:19mMaster General properties of epoxidation. with a bite sized video explanation from Johnny
Start learning