Textbook Question
Draw the correct structure from the following IUPAC names:
(b) 4-methoxybut-1-yne
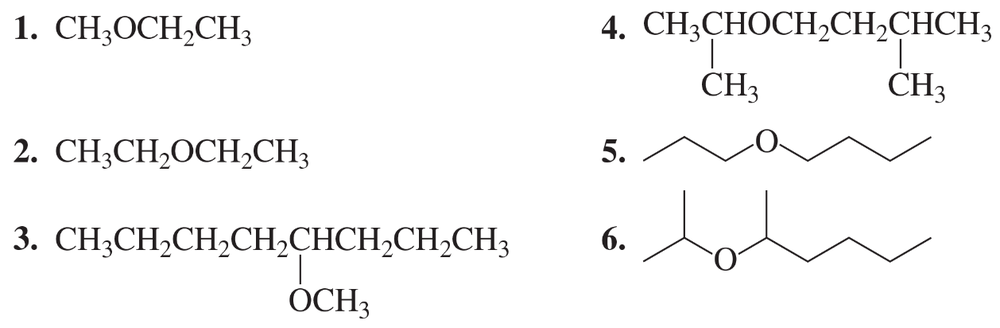
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 1:32m
1:32mMaster How to name ethers using the common naming system. with a bite sized video explanation from Johnny
Start learning