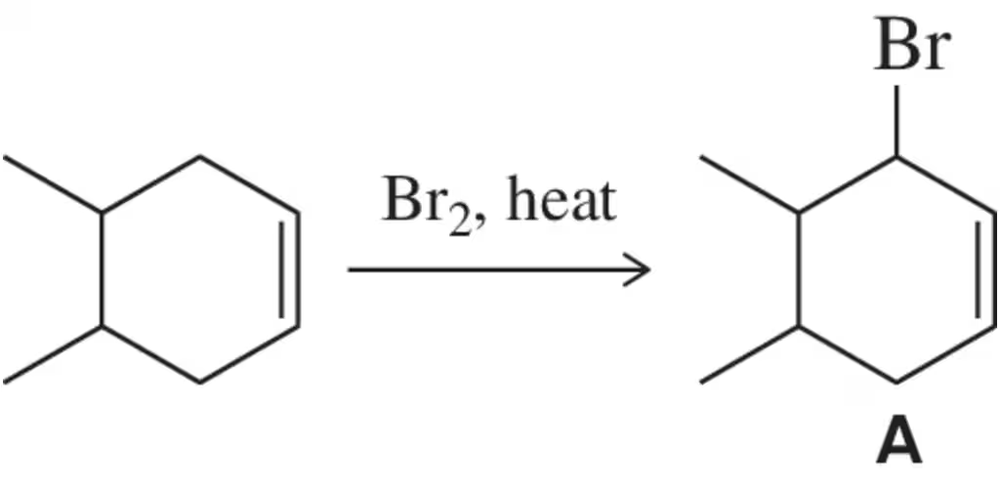
In the presence of a small amount of bromine, cyclohexene undergoes the following light-promoted reaction:
d. Explain why cyclohexene reacts with bromine much faster than cyclohexane, which must be heated to react.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 6:15m
6:15mMaster The general mechanism of Allylic Halogenation. with a bite sized video explanation from Johnny
Start learning