Predict the products (if any) of the following acid–base reactions.
(d) α-bromopropionic acid + sodium propionate
(e) benzoic acid + sodium phenoxide
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: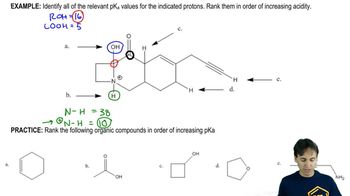


 3:15m
3:15mMaster Why we need factors affecting acidity and when to use them. with a bite sized video explanation from Johnny
Start learning