a. How could each of the following compounds be prepared from a hydrocarbon in a single step?
b. What other organic compound would be obtained from each synthesis?
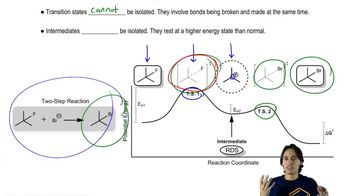
2.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: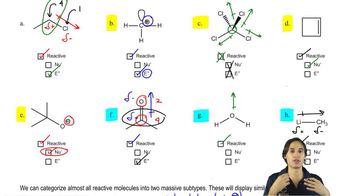
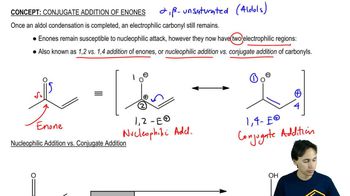

 12:6m
12:6mMaster Conjugated Hydrohalogenation - General Mechanism with a bite sized video explanation from Johnny
Start learning