Show how each of the following compounds can be synthesized from benzene:
d. anisole
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
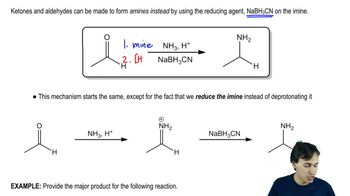

 2:49m
2:49mMaster Aromatic synthesis starting with benzene/benzene derivatives with a bite sized video explanation from Johnny
Start learning