Identify whether each of the following reactions proceed by an SN1, SN2, E1, or E2 mechanism.
(c)
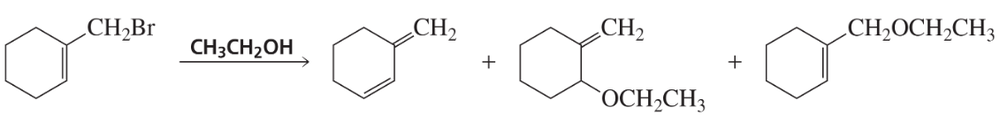
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 2:27m
2:27mMaster Overview of the flowchart. with a bite sized video explanation from Johnny
Start learning