Ciprofloxacin is a member of the fluoroquinolone class of antibiotics.
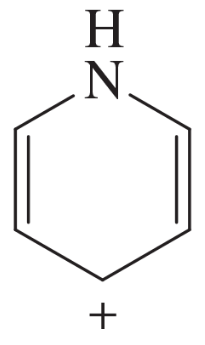
(b) Which nitrogen atoms are basic?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 2:04m
2:04mMaster Heterocycles - Which lone pairs react? with a bite sized video explanation from Johnny
Start learning