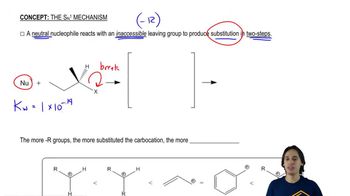
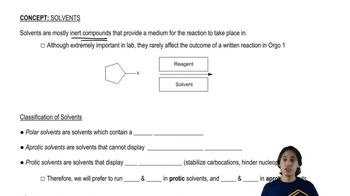
When tert-butyl bromide is heated with an equal amount of ethanol in an inert solvent, one of the products is ethyl tert-butyl ether.
b. What happens to the rate if the concentration of tert-butyl bromide is tripled and the concentration of ethanol is doubled?
c. What happens to the rate if the temperature is raised?