(a) Suggest an arrow-pushing mechanism for the mutarotation of α-d-glucose to β-d-glucose in the presence of acid. Acid isn’t necessary, but it does increase the rate of the process.
(b) What is the role of acid in your mechanism?
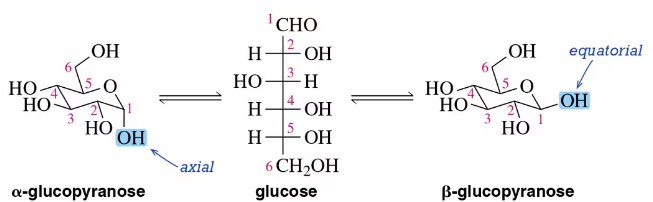
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: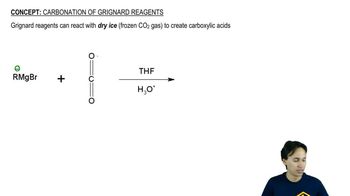
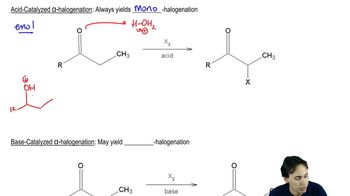
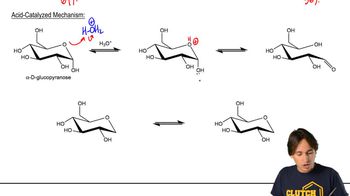

 8:17m
8:17mMaster Mutorotation and Optical Activity with a bite sized video explanation from Johnny
Start learning