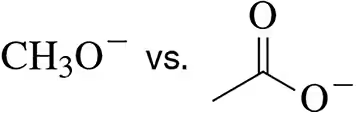
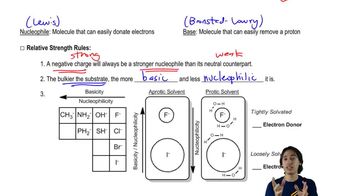
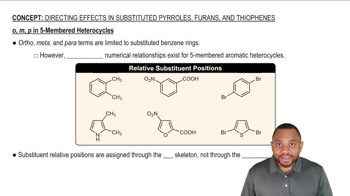
In addition to radicals, anions, and cations, a fourth class of reactive intermediates is carbenes. A neutral species, the simplest carbene has a molecular formula of CH2.
(d) Carbenes are also Lewis bases. Based on your answers to (a) and (b), explain this observation.