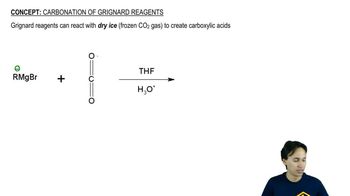
We discuss the reaction of Grignard reagents (organomagnesium compounds) to ketones in Chapter 17. Mechanistically, the reaction proceeds by the nucleophilic addition of a methyl carbanion to the electrophilic carbon of the carbonyl, breaking the C―Oπ bond, resulting in an alkoxide intermediate that is subsequently protonated to produce the 3° alcohol.
(a) Why does this reaction produce a racemic mixture of 3° alcohols?