Each of the following heterocycles includes one or more nitrogen atoms. Classify each nitrogen atom as strongly basic or weakly basic, according to the availability of its lone pair of electrons.
(d)
(e)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: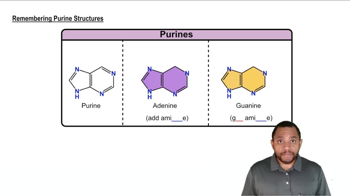


 2:04m
2:04mMaster Heterocycles - Which lone pairs react? with a bite sized video explanation from Johnny
Start learning