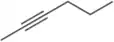
Answer Problem 39 , parts a–h, using 2-butyne as the starting material instead of propyne.
c. Br2 (1 mol)/CH2Cl2
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 2:15m
2:15mMaster Double halogenation of alkynes. with a bite sized video explanation from Johnny
Start learning