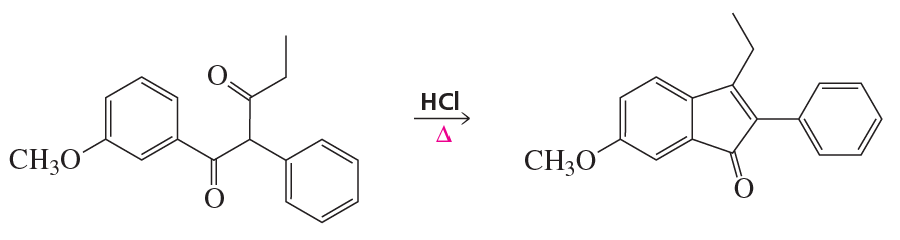
cis-4-Bromocyclohexanol and trans-4-bromocyclohexanol form the same elimination product but a different substitution product when they react with HO−.
c. How many stereoisomers does each of the elimination and substitution reactions form?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 0:38m
0:38mMaster Intro to Substitution/Elimination Problems with a bite sized video explanation from Johnny
Start learning