a. Which of the species have bond angles of 109.5°?
b. Which of the species have bond angles of 120°?
H2O H3O+ +CH3 BF3
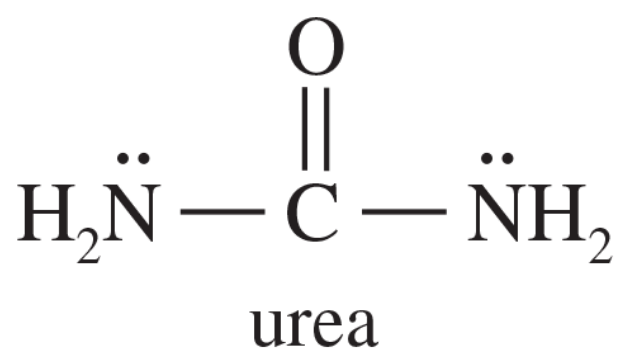
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 7:44m
7:44mMaster Molecular Geometry Explained. with a bite sized video explanation from Johnny
Start learning