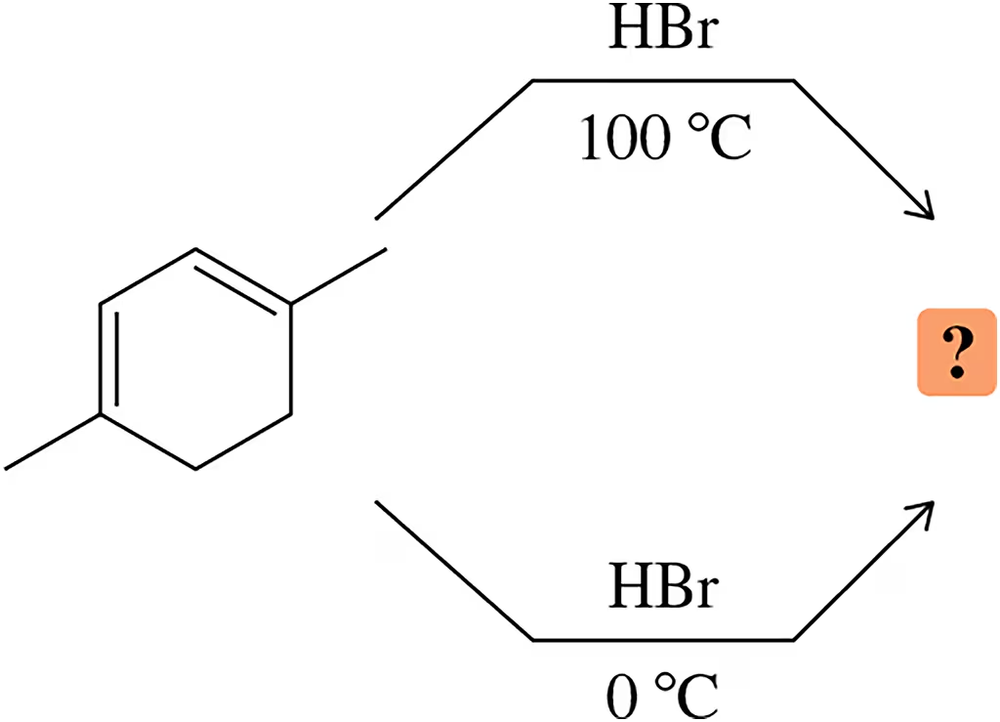
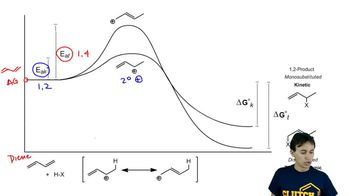
The experiment shown next and discussed in Section 8.13 shows that the proximity of the chloride ion to C-2 in the transition state causes the 1,2-addition product to form more rapidly than the 1,4-addition product.
a. Why was it important for the investigators to know that the preceding reaction was being carried out under kinetic control?