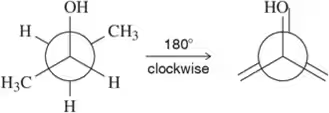
Using the Newman projections shown, draw each molecule in its line-angle drawing with all hydrogens and substituents shown. [Carbon b is behind carbon a in these structures.] Wedges and dashes should be used to indicate whether a substituent is coming out of, or going into, the plane of the page.
(b)