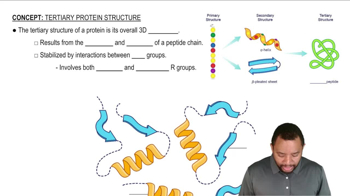
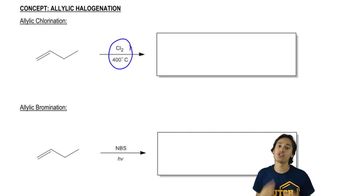
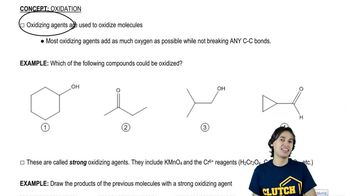
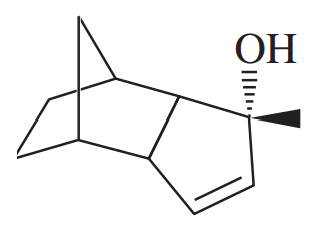
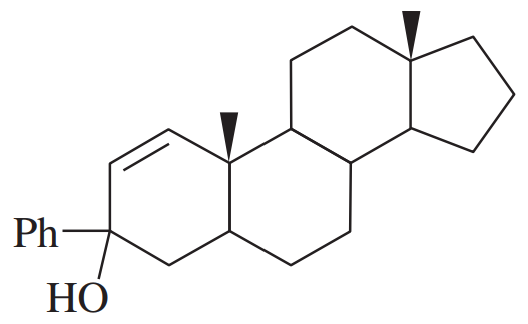
Predict the products of the reactions of the following compounds with:
1. chromic acid or excess sodium hypochlorite with acetic acid.
2. PCC or NaOCl (1 equivalent) with TEMPO.
e. cyclohexane
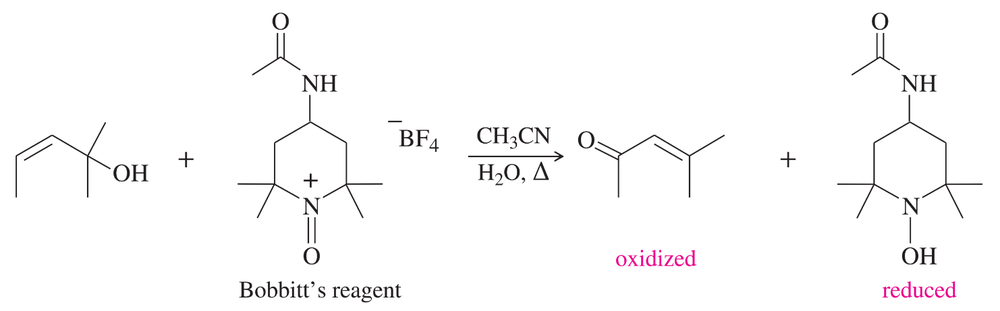
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: