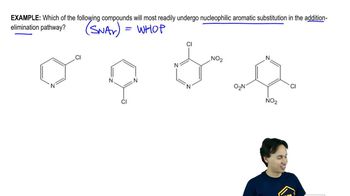
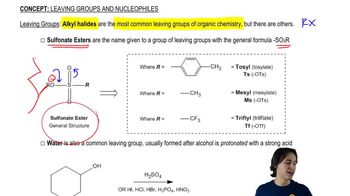
Propose mechanisms and show the expected products of the following reactions.
(c) p-nitrobromobenzene + methylamine (CH3–NH2)
(d) 2,4-dinitrochlorobenzene + excess hydrazine (H2N–NH2)
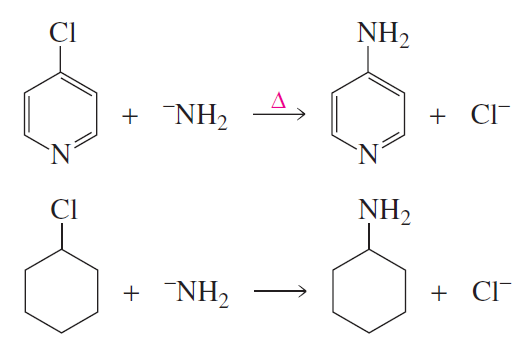
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: