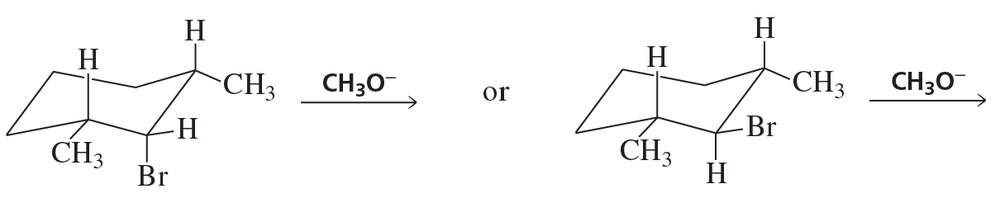
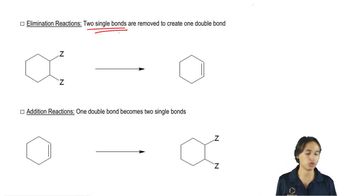
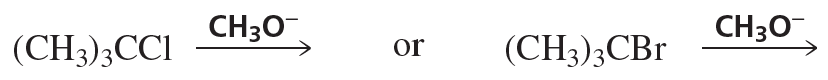a. Design an alkyl halide that will give only 2,4-diphenylpent-2-ene upon treatment with potassium tert-butoxide (a bulky base that promotes E2 elimination).
b. What stereochemistry is required in your alkyl halide so that only the following stereoisomer of the product is formed?