Draw and name the products of nitric acid oxidation of
(a) D-mannose
(b) D-galactose
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
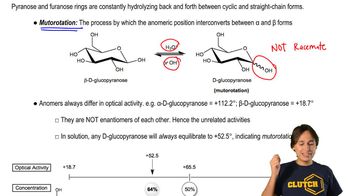
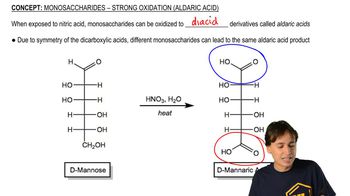

 3:10m
3:10mMaster Monosaccharides - Strong Oxidation (Aldaric Acid) with a bite sized video explanation from Johnny
Start learning