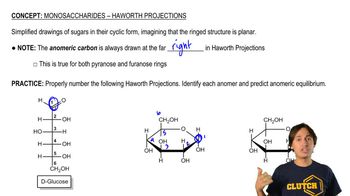
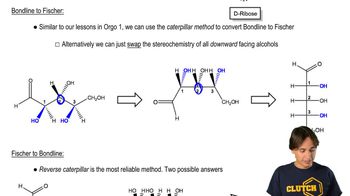
Glucose is the most abundant monosaccharide. From memory, draw glucose in
(a) the Fischer projection of the open chain.
(b) the most stable chair conformation of the most stable pyranose anomer.
(c) the Haworth projection of the most stable pyranose anomer.