Show a mechanism for the following elimination reactions. Label the mechanism as E1 or E2.
(c)
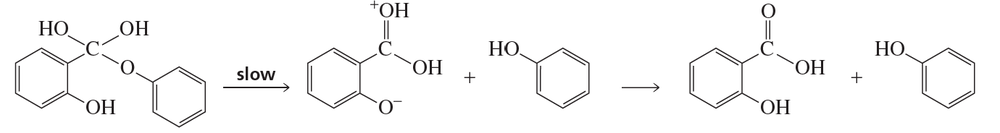
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 0:38m
0:38mMaster Intro to Substitution/Elimination Problems with a bite sized video explanation from Johnny
Start learning