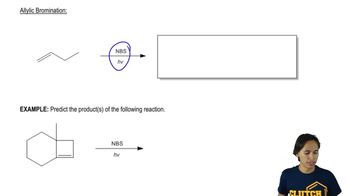
The first step of a reaction called electrophilic aromatic substitution is as follows:
If this step is rate-determining for the overall reaction, which benzene derivative would you expect to react most quickly? Which would react most slowly?
(a)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 4:29m
4:29mMaster Activity and Directing Effects with a bite sized video explanation from Johnny
Start learning