Tyramine is an alkaloid found in mistletoe and ripe cheese. Dopamine is a neurotransmitter involved in the regulation of the central nervous system.
b. How can dopamine be prepared from tyramine?
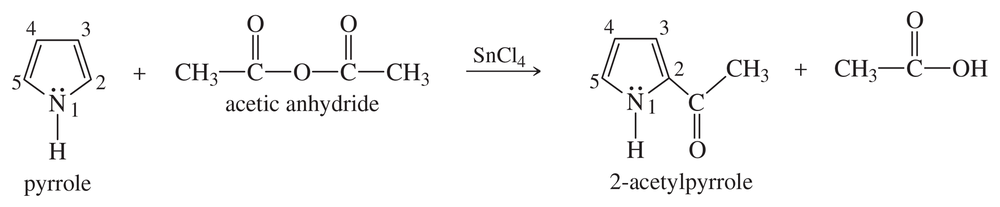
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 2:49m
2:49mMaster Aromatic synthesis starting with benzene/benzene derivatives with a bite sized video explanation from Johnny
Start learning