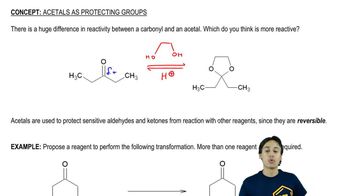
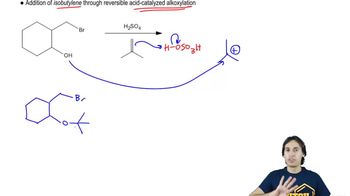
Draw the following sugar derivatives.
(a) methyl β-D-glucopyranoside
(b) 2,3,4,6-tetra-O-methyl-D-mannopyranose
(c) 1,3,6-tri-O-methyl-D-fructofuranose
(d) methyl 2,3,4,6-tetra-O-methyl-β-D-galactopyranoside
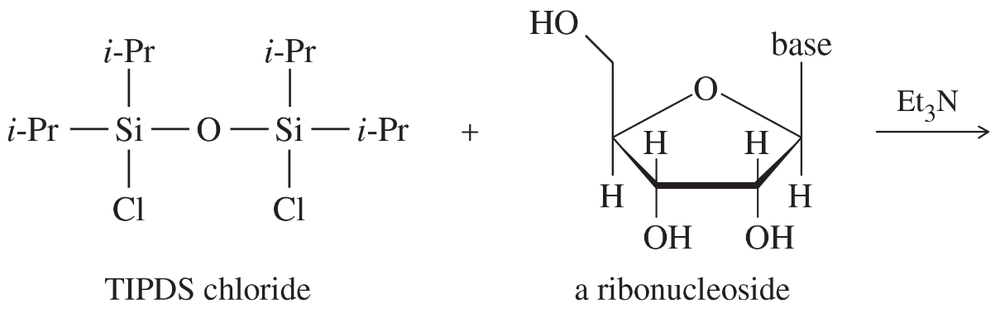
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: