Use a Frost circle diagram to construct the molecular orbital diagram for the molecules shown. Would you expect them to be aromatic or antiaromatic?
(b)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: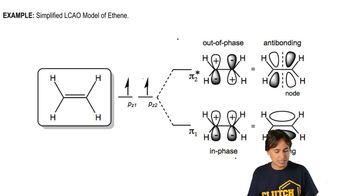


 12:16m
12:16mMaster Inscribed Polygon Method with a bite sized video explanation from Johnny
Start learning