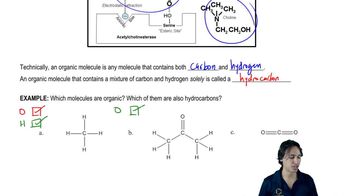
What is the molecular formula for each of the following?
a. a 4-carbon hydrocarbon with two bonds and no rings
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: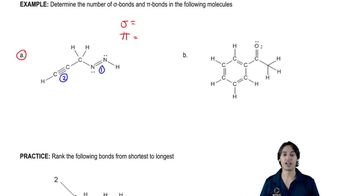

 2:39m
2:39mMaster The difference between saturated and unsaturated molecules. with a bite sized video explanation from Johnny
Start learning