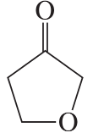
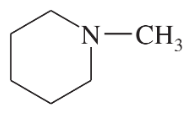
Circle the functional groups in the following structures. State to which class (or classes) of compounds the structure belongs.
a. CH2=CHCH2COOCH3
b. CH3OCH3
c. CH3CHO
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 1:49m
1:49mMaster Why we need functional groups. with a bite sized video explanation from Johnny
Start learning