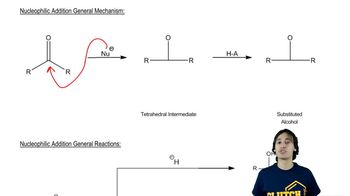
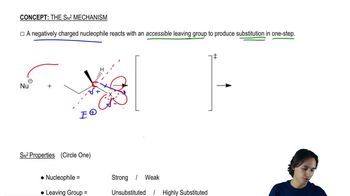
Under appropriate conditions, (S)-1-bromo-1-fluoroethane reacts with sodium methoxide to give pure (S)-1-fluoro-1-methoxyethane.
a. Why is bromide rather than fluoride replaced?
b. Draw perspective structures (as shown on the previous page for 2-bromobutane) for the starting material, the transition state, and the product.
c. Does the product show retention or inversion of configuration? d. Is this result consistent with reaction by the SN2 mechanism?