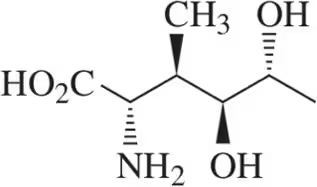
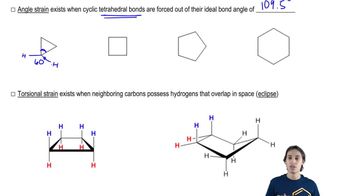
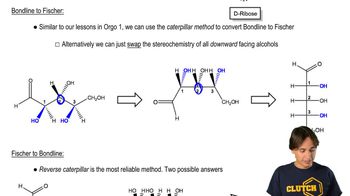
For each Fischer projection,
1. make a model.
2. draw the mirror
3.. determine whether the mirror is the same as, or different from, the original structure.
4. draw any mirror planes of symmetry that are apparent from the Fischer projections.
(c)
(d)