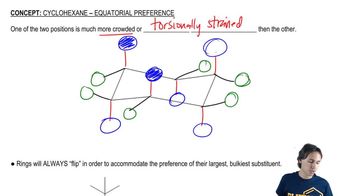
Draw the two chair conformations of each compound, and label the substituents as axial and equatorial. In each case, determine which conformation is more stable.
a. cis-1-ethyl-2-isopropylcyclohexane
b. trans-1-ethyl-2-isopropylcyclohexane
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 4:02m
4:02mMaster Equatorial Preference with a bite sized video explanation from Johnny
Start learning