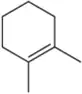
Provide arrow-pushing mechanisms for Assessments 9.10(b) and 9.10(c) that rationalize the regioselective and stereospecific formation of each halohydrin.
(b)
(c)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 1:44m
1:44mMaster General properties of halohydrin formation. with a bite sized video explanation from Johnny
Start learning