Textbook Question
(b) Explain why m-xylene undergoes nitration 100 times faster than p-xylene.
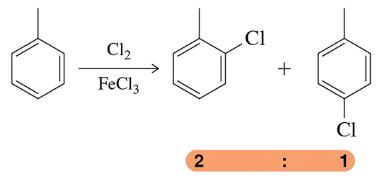
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 4:42m
4:42mMaster Ortho, Para major products with a bite sized video explanation from Johnny
Start learning