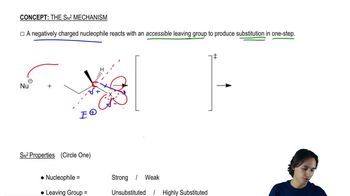
Under certain conditions, the reaction of 0.5 M 1-bromobutane with 1.0 M sodium methoxide forms 1-methoxybutane at a rate of 0.05 mol/L per second.
c. Show another SN2 reaction using a different combination of an alkoxide and an alkyl bromide that also produces 1-methoxybutane.