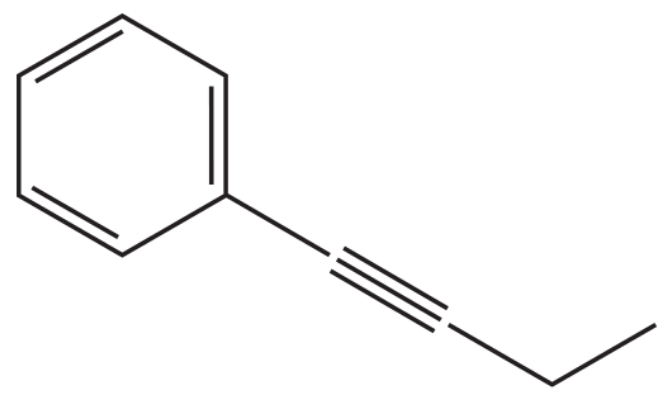
Suggest two coupling partners that could be used to synthesize the compounds shown making the indicated C―C bonds.
(a)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 2:54m
2:54mMaster Sonogashira Coupling Reaction with a bite sized video explanation from Johnny
Start learning