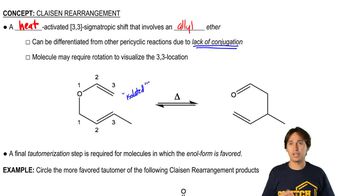
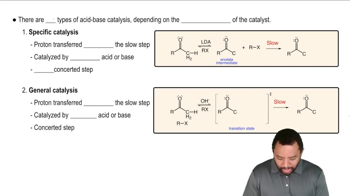
Alcohols combine with ketones and aldehydes to form interesting derivatives, which we will discuss in Chapter 18. The following reactions show the hydrolysis of two such derivatives. Propose mechanisms for these reactions.
(b)
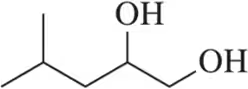
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: