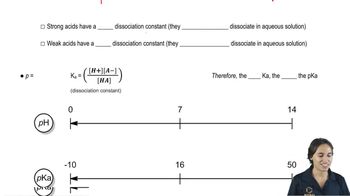
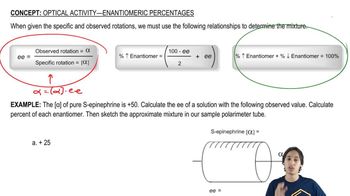
The A value of a substituent on a cyclohexane ring is essentially the ∆G° for a substituent going from the equatorial to the axial position in a chair–chair interconversion. Because most substituents prefer to be in the equatorial position, A values are, by definition, positive numbers. Use the table of A values to calculate ∆G° and Keq for the chair–chair interconversions shown.
(d)