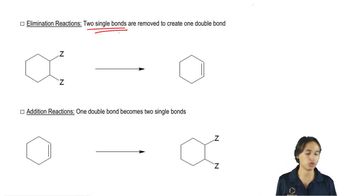
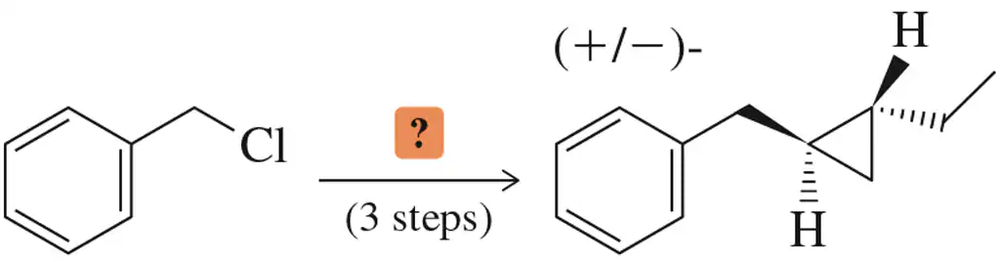
Develop syntheses for the following compounds, using acetylene and compounds containing no more than four carbon atoms as your organic starting materials.
(a) 3-methylnon-4-yn-3-ol (“3-ol” means there is an OH group on C3.)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: