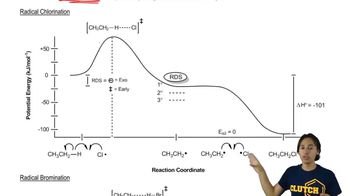
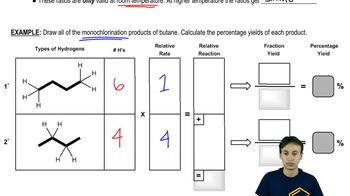
What is the major product obtained from treating an excess of each of the following compounds with Cl2 in the presence of ultraviolet light at room temperature? Disregard stereoisomers.
a.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: