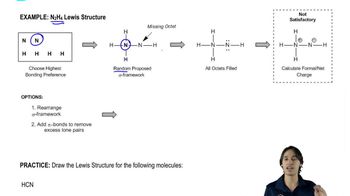
1. Draw the Lewis structure.
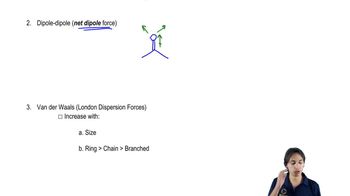
2. Show how the bond dipole moments (and those of any nonbonding pairs of electrons) contribute to the molecular dipole moment.
3. Estimate whether the compound will have a large, small, or zero dipole moment.
g. HCN
h. CH3CHO
i. H2C=NH