Predict the product(s) that would result when the alkenes are allowed to react under the following conditions: (vii) 1. mCPBA 2. H2SO4, H2O (viii) 1. O3 2. CH3SCH3
(l)
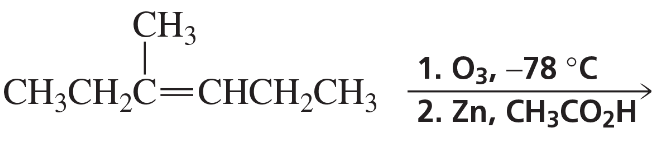
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 6:30m
6:30mMaster General properties of ozonolysis. with a bite sized video explanation from Johnny
Start learning