Choose the member of each pair that will react faster by the SN1 mechanism.
a. 1-bromopropane or 2-bromopropane
b. 2-bromo-2-methylbutane or 2-bromo-3-methylbutane
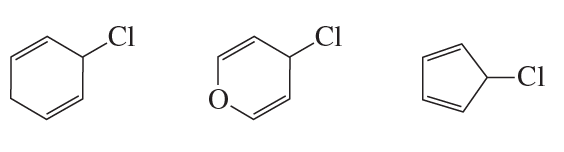
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 1:49m
1:49mMaster Drawing the SN1 Mechanism with a bite sized video explanation from Johnny
Start learning