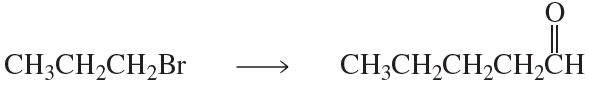
Beginning with the molecules on the left, provide a synthesis of the molecule on the right. The ideal number of steps is indicated over the reaction arrow, although there may be alternate routes worth considering.
(b)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 3:19m
3:19mMaster Sodium Alkynide Alkylation with a bite sized video explanation from Johnny
Start learning