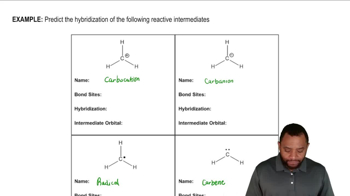
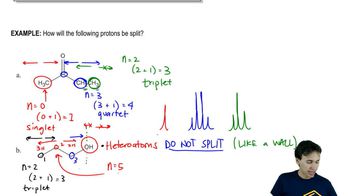
Suggest an arrow-pushing mechanism that accounts for the formation of the following Lewis acid–Lewis base complexes. Label the Lewis acid and Lewis base in each.
(a)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 2:49m
2:49mMaster The Lewis definition of acids and bases. with a bite sized video explanation from Johnny
Start learning