Show how you would accomplish the following synthetic conversions. You may use any additional reagents and solvents you need.
(a)
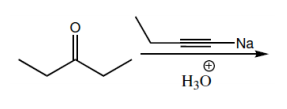
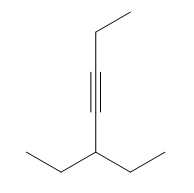



 Verified step by step guidance
Verified step by step guidance
 3:57m
3:57mMaster Nucleophilic Addition on Ketones and Aldehydes with a bite sized video explanation from Johnny
Start learning
