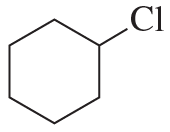
What is the major elimination product obtained from an E2 reaction of each of the following alkyl halides with hydroxide ion?
a.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 1:18m
1:18mMaster Defining Zaitsev’s Rule with a bite sized video explanation from Johnny
Start learning